Our key takeaways from the BICO 2021 partnership conference
On the 11th and 12th of November, we did not hesitate for too long whether to focus on our lab work or attend the BICO Partnership Conference talks live. Those were two inspiring days for any tissue engineer.
We heard Dr. Cato Laurencin stress that in tissue engineering, cells that are efficient at producing matrix molecules are as important, if not more important than cells that proliferate potently. Dr. Carol Treasure challenged us to develop procedures and tissue engineering products that use no animal-derived products whatsoever just like they do at XCellR8. A number of presenters reminded us of the painfully low predictability that animal models give us for whether a drug candidate will be efficacious in humans or not. Dr. Tom Villani convinced us that in spite of the race to recapitulate the complexity of a tissue, an organ or even a body, simple tissue models will always be useful because they make it easier to draw causal conclusions for the biochemical effects of a drug, for instance. Dr. Peter Yeow got us excited about DeepMind’s AI, Alphafold but that is a topic for another post.
We paid closer attention to the presentation of Dr. Alice E. White whose work was built upon a number of key achievements in tissue engineering which are relevant to the products we have been developing. These achievements included the understanding of the effect of (bio)material stiffness on cell functioning, the resolution achieved by stereolithographic biofabrication methods, and the ability to differentiate reproducibly human-induced pluripotent stem cells. While Dr. White’s work was on creating a platform for the high-throughput and real-time monitoring of in vitro heart muscle tissues, we were intrigued by the work which preceded it.
Mesenchymal stem cells seeded on an array of pillars with various heights of 0.97 microns (left), 6.1 microns (middle), and 12.9 microns (right). Fu et al. (2010)
There has been steadily accumulating evidence that the stiffness of the material with which cells interact affects their fate. In tissue engineering, the general rule is that the (bio)material needs to reproduce the mechanical properties of the native tissue from which the cells originate. Furthermore, stiffness affects the differentiation of mesenchymal (adult-derived) stem cells. When cultured in the same (bipotential) medium, stem cells start turning into a bone cell (osteogenic differentiation) if the substrate is stiff (often incorrectly called hard) or into a fat cell (adipogenic differentiation) if the substrate is compliant (often incorrectly called soft). However, a limitation of most of the studies showing this effect is a number of uncontrolled confounding variables such as concentration of cell-binding sites, material porosity, surface wettability, and differences between microscopic molecular stiffness and macroscopic material stiffness. A clever approach reported in Nature Methods in 2010 addressing these limitations consisted of creating pillars with varying height which enabled the researchers to maintain everything else constant while varying only the substrate stiffness. The shorter the pillar, the stiffer the substrate. The authors showed that the substrate stiffness controls cell morphology, focal adhesions, cytoskeletal contractility and stem cell differentiation. The shorter the pillars, the more likely it was that the stem cells would start along the osteogenic differentiation path, while the taller pillars “guided“ the cells along the adipogenic path.
Fluorescent microscopy images showing cells (green) and fibronectin cell-attachment sites (red). Klein et al. (2011)
The process of fabrication of the substrate in the above paper included a classical photolithography approach which has been perfected for the needs of the semiconductor industry but it is cumbersome for tissue engineering purposes. Instead, stereolithographic 3D printing methods are preferred since they enable much faster prototyping with very high resolution and are already being optimized for biofabrication. A new and exciting stereolithographic method is the two-photon polymerization 3D printing which Dr. White showed to be able to achieve a resolution as low as 100 nm as implemented in the Nanoscribe’s system. This resolution enables material engineering at scale lengths relevant to the cell-material interactions. Thus, tissue engineers can perform controlled experiments to reveal the cues guiding cell behavior. In one such study, researchers combined two materials of which one did and the other did not allow for cell attachment. Consequently, printing complex 3D structures enabled the full control over the cell shape in three dimensions. This approach has since been used in numerous studies investigating the spatial 3D distribution of various functionalities which we will explore deeper in a future post.
Note: The video from the original publication was heavily edited, including the removal of some of the frames, to reduce the size of the shown file for webpage performance purposes.
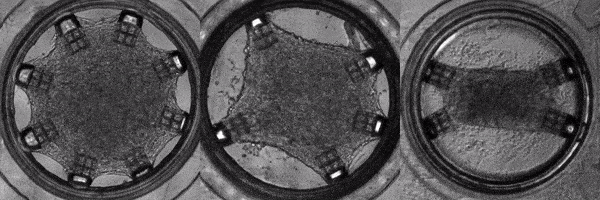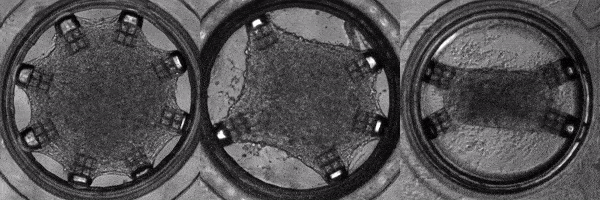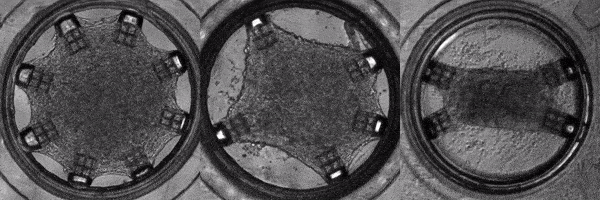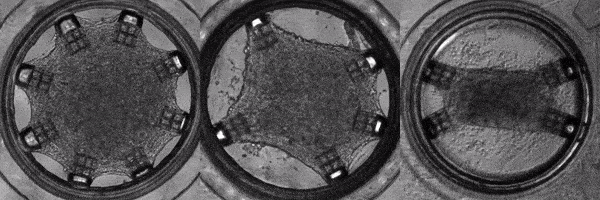
Human induced pluripotent stem cells (hiPSCs) are adult (skin or blood) cells turned into stem cells which can be further differentiated into many of the cells of the human body. The generation of hiPSCs forever changed the field of regenerative medicine by providing an appealing alternative to embryonic stem cells. Furthermore, as iPSCs are derived from patients, diseases can be modeled on a patient-by-patient basis enabling the screening of pharmacological agents to find the ideal one for each individual. In this light, Cell-MET develops tissue-engineering principles to create technologies for growing clinically significant heart muscle tissues. This goal relies on established efficient protocols for the differentiation of hiPSCs into heart muscle cells (cardiomyocytes) and their subsequent maturation. According to one differentiation protocol, using the right sequence of chemical treatments, hiPSCs can be turned into beating cardiomyocytes within 9 to 14 days. Unfortunately, according to a recent review, the hiPSC-derived cardiomyocytes remain largely immature as compared to human adult cardiomyocytes which hinders their usage in pharmacological and toxicological screening, as well as cardiovascular disease modelling. To address this issue effectively, in a recently published work, Dr. White’s group and their collaborators have demonstrated a platform for for the investigation of the maturation of heart tissue under mechanical, electrical, and chemical conditioning.
Our interest in the topics covered herein is motivated by the products we have been developing. For instance, the GelMA hydrogels are well known for their malleability in terms of mechanical properties which can be achieved in various ways. Furthermore, we are currently developing two collagen-based formulations whose mechanical properties will be similarly tunable. On the other hand, we have also been developing biocompatible resin formulations suitable for two-photon polymerization stereolithography. Finally, keeping in mind the importance of stem cells, we have initiated a collaboration to validate our products in the context of regenerative medicine and the creation of an in vitro tissue model. Stay tuned for both product updates and announcements on our collaborations. You can also collaborate with us to create the next clinically relevant tissue model as a student or as a partner.