Bioprinting is accessible… today!
Below, we provide arguments for why it is time that there be at least one biofabrication device, if not a whole biofabrication lab, in every medical and biology (and even chemistry) university, faculty, or institute. No matter whether in the USA, the Netherlands, China, Germany or here in Bulgaria! No forward-thinking head of an institution could have missed the explosive development in the fields of bioengineering, tissue engineering, and regenerative medicine. None of these leaders can justify indifference to the forthcoming tremendous societal benefits of this scientific and engineering development. By extension, contributing to the foundations of the field or developing applied solutions seems imperative for anyone who values the well being of their loved ones. The matter seems even more pressing considering Europe’s desire to completely eliminate animal testing, the low predictive power of current preclinical studies, the limitation of organ donation and organ transplantation, and the prevalent impact of chronic diseases on well-being, including cancer, heart disease, osteoporosis and others. This is probably why market research reports have predicted a compound annual growth rate (CAGR) of 20.4% and 35.4% for the period 2019 to 2024 which project the market to reach $1.65 billion and $1.4 billion, respectively.
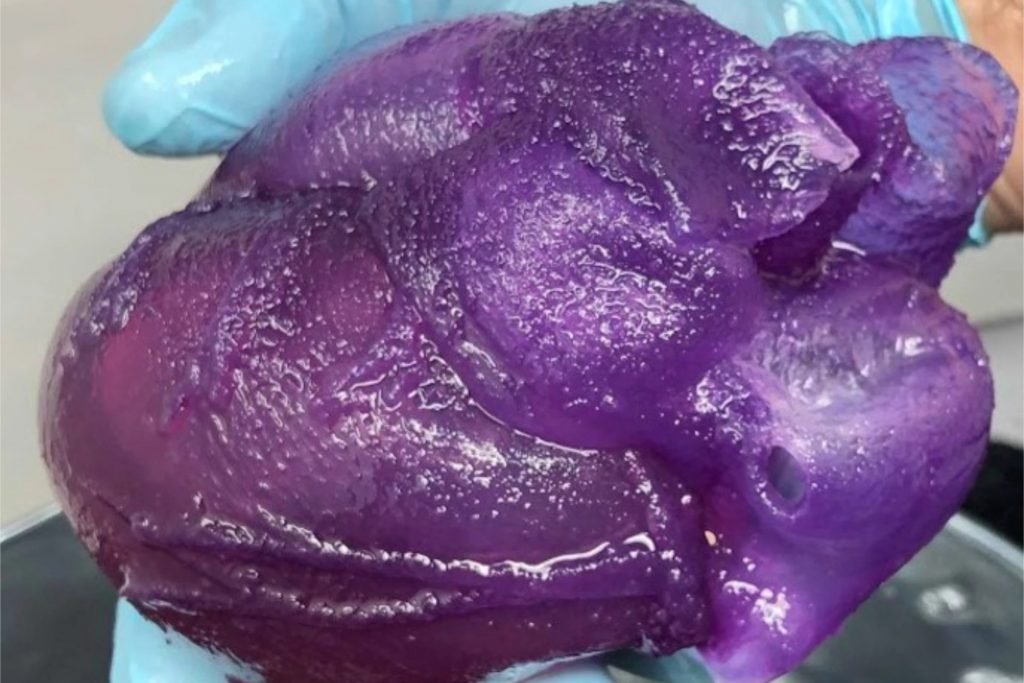
Full-sized heart bioprinted on a DIY bioprinter reportedly costing as little as ~$50.
But do young students, engineers and researchers care? Do they care about being able to print an organ, just because they could? Do students care about animal wellbeing and would like to see the development of technologies replacing animal testing? Do aspiring surgeons care how many real-life-like organs and tissues they would handle before going for the real deal? Do surgeons care if they could print a tissue onto or into a patient? Of course, at MatriChem, we are biased and these sound like rhetorical questions to us but they are also real questions we ask ourselves when we look around and see the attitude of the general public towards science and new disruptive technologies. The question that really excites us, however, is what would students and young inventors and entrepreneurs dream about once bioprinting becomes a routine every-day activity?
But isn’t it expensive to get one of these biofabrication devices, you may ask? Don’t you need a very specific and deep expertise to operate them and be able to contribute meaningfully to the field, you may insist? The answer to the first question is paradoxically both of course it is and not at all. Here, we provide evidence for why it is not expensive and why now is the best time to take advantage of it. As to the second question, the answer is yes, definitely, and we as a society need to spread this expertise as soon and as quickly as possible so that we may hope to reap the benefits in the foreseeable future. At the same time, the longer we fail to develop the required expertise and teach it to the current university students, the more difficult it might become to bridge the ever accumulating technological and technical expertise divide between the various nations.
It is not expensive to get a biofabrication device. It has never been! But before delving into the details of why, we should clarify that developing anything close to an applied solution or a product intended for the market, and specifically the patient, requires high quality (and sometimes quite expensive) certified tools and consumables. They provide a way to ensure the necessary consistency from one product to the next. Their high cost, however, makes them rather unsuitable for training young and inexperienced students, as well as for “freestyle“ experimentation and exploration of equipment limitations and capabilities. Furthermore, they lack in flexibility and cannot be easily modified. These limitations may prevent the development of deep knowledge and expertise in the future mechanical, electrical, software, chemical, and tissue engineers. As a result, these minds are less likely to contribute key innovative solutions and products.
On the other hand, low cost and open source options to create a DIY biofabrication devices are suitable to not only ”play around” or train inexperienced students but indeed, to perform high quality science as the number of papers published with results generated using a DIY biofabrication device proves. Notably, this is not a field dominated by big industrial players (yet) but it is a field of constant innovation within each of the pillars of biofabrication, i.e. the software, the hardware, the materials, and the cells. This situation is ideal for a novice but inspired interdisciplinary team with a clear vision and appropriate level of engineering training to quickly catch up with some of the most recent achievements and consequently contribute key novel solutions and products. Our expectation, however, is that in terms of market opportunities, the situation will soon change.
In this piece, inspired by recent publications by Tong and colleagues, Garciamendez-Mijares and colleagues and Bharadwaj and Verma, we review the available affordable bioprinters as well as the open source information enabling the construction of DIY bioprinters. The motivation behind this piece is to facilitate the technological democratization by curating the information allowing research groups and inspired hackers and makers anywhere in the world to fabricate real-life-like organs for a fraction of the cost. At the end, we discuss some of the typical challenges before any novice team excited to delve into the world of bioprinting.
Affordable and DIY extrusion bioprinters
In extrusion bioprinting, the bioink is extruded via a nozzle by mechanical or pneumatic means as a continuous filament onto a platform. The nozzle is moved around over the platform to create the designed pattern. By depositing consecutive layers with appropriately planned paths, a final 3D tissue model is created. In pneumatic extrusion, compressed air ejects the bioink out of the nozzle where the flow rate is regulated by the air pressure. On the other hand, in mechanical extrusion, the process is typically driven by a stepper motor, which ultimately provides linear actuation of a plunger pushing the bioink out through the nozzle.
An example of a mechanical extrusion bioprinter utilizing a 10 ml syringe as implemented in Tissue Scribe.
The price range of affordable extrusion bioprinters is from ~$1700 to $10,000. The most economical ones are the single-nozzle mechanical extrusion-based printing systems. One such bioprinter is Tissue Scribe which costs $1699. Instead of the typical cartridge, the system utilizes the widely available syringes. Furthermore, the bioprinter can heat the syringe and the platform (a.k.a. print bed) to maintain a cell friendly environment or to modify material viscosity when needed. The flexibility of such systems is an important selling point. For instance, the Tissue Scribe bioprinter can be converted to a conventional polymer melt 3D printer for an additional cost of $1000 significantly expanding its applicability.
Another even more flexible bioprinter is with a price tag of ~$2500. Hyrel 3D’s Engine SR has been created with modularity in mind and offers the option to select modular print heads from a wide range of options, including cold/warm flow heads, syringe heads, and hot flow heads. In the context of bioprinting, the most relevant are the Syringe Dispensing System (SDS) and the Crosslinking Dispensing System (CDS), the latter of which provides the option to crosslink light-sensitive inks with light of various wavelengths from 280 to 450 nm. Of course, each head raises the overall cost of the bioprinter where an SDS head adds $400, while a CDS head adds $550 to the total cost. In comparison, the Engine HR, which is specifically designed for the bioprinting community, is a more compact but also more expensive version (~$8000) of the SR model.
According to the research of Tong and colleagues, one of the top 5 most affordable pneumatic bioprinters on the market is offered by Axolotl Biosystems. Similar to Hyrel 3D’s systems, Axolotl’s machines also come with sockets for interchangeable print heads. Notably, the basic unit includes a print head which can be heated up to 210°C, a print bed which can be heated up to 60°C, and a UV crosslinking tool head. The working pressure range is from ~14 to 145 psi (from ~27.5 kPa to 1 MPa) and the system has the ability to autocalibrate its heads. For additional cost, exciting options such as compatible melt electrowriting, cooled print head, as well as a cooled print bed can be implemented.
At a starting price of ~$5000, BIOBOT BASIC offers a non-standard approach to bioprinting with a polar coordinate bioprinter. Uniquely, it comes with a revolver-inspired pneumatic extrusion head allowing the printing of up to five different biomaterials without the need to interrupt the extrusion process by manually switching bio-ink cartridges. The working pressure range of the system is from 0.5 to 85 psi (3.5 kPa to ~590 kPa).
The price range of DIY extrusion bioprinters starts from ~$50 but their realization requires the involvement of a technically savvy person. The typical strategy is to utilize a filament-forming fabrication (FFF or FDM) 3D printer to print the necessary parts, and by purchasing some additional but common parts, transform the same machine into an extrusion bioprinter. In fact, there is a number of peer reviewed publications, including in a journal from the Nature Portfolio, which have detailed the successful implementation of this strategy and their subsequent utilization for biofabrication. Recent reviews from Garciamendez-Mijares and colleagues and Bharadwaj and Verma provide plenty of details for the interested reader.
One such study from 2016 showed how a commercially available desktop 3D printer could be modified to bioprint viable cells in a bioink. A MakerbBot Replicator Experimental 3D Printer was modified to include a syringe injection unit (SIU). Before the modification, the SIU’s syringe attachment, housing, guides, and motor mounts were printed on the Rep2X 3D printer. Importantly, the modification was such that all of the parts that come in contact with bioinks were removable and could be autoclaved or could be disposed of. The authors reported the printing of cartilage cells and showed their proper functioning.
Later in the same year, Reid and colleagues made the strategy even more accessible in an open access publication. Aiming to circumvent the high-price barrier to entry of conventional bioprinters, they reported a design of components for the adaptation of an inexpensive ‘off-the-shelf’ commercially available 3D printer, Felix 3.0. Once again, before the modification, many of the necessary parts were printed by the 3D printer using polylactic acid (PLA) or acrylonitrile butadiene styrene (ABS). Impressively, the system was able to achieve single cell print resolutions within 50 μm while minimizing unwanted impact on viability of stem cells, which were also shown to maintain their pluripotency, i.e. stemness. Also, excluding the cost of the Felix 3.0 3D printer, the upgrade required less than $200.
Another publication described a system capable of printing a variety of bio-materials through a multi-channel 3D bioprinting technology. Reminiscent of the BIOBOT BASIC, the devised system employed a carousel dispensing system with a rotary actuator enabling it to print with up to five heads without the need to interrupt the printing process to switch from one material to another. In contrast, however, it comprised of pneumatic and mechanical extrusion for continuous dispensing and needle valve inkjet for drop-on-demand dispensing accommodating a range of possible material properties. This achievement is impressive given that such a range of capabilities can be found only in recent generations of commercially available bioprinters.
In 2018, Pusch and colleagues reported a large volume syringe pump extruder after modification of PrintrBot Simple Metal 3D printer. The authors have reported the cost of the hardware to be as low as $49.27. To date, this is the most affordable bioprinting-capable system. Excitingly, with its open source software and hardware, this system makes bioprinting accessible to anyone interested by practically removing the cost barrier. Still, there is an ongoing effort by a number of groups around the world to create other affordable and open source bioprinting systems with a most recent report at the end of 2021. The notable feature of this system is its ability to automatically replace the printing head. The head changer can retrieve up to four syringes enabling another approach for multimaterial bioprinting.
Handheld bioprinter used to print muscle tissue into living mice.
An exciting class of affordable and open source extrusion bioprinters is the handheld bioprinters. Handheld bioprinters enable in situ (or in vivo) biofabrication, i.e. printing bioinks directly onto a patient, due to their small size, ergonomic design, simplicity, and convenience. One example is a handheld bioprinter reportedly costing as little as $121. A basic handheld bioprinter consists of a body, printing nozzle, one or two bioink cartridges, device regulatory electronics, and motorized or pneumatically controlled system. Unlike mechanical arm bioprinters, handheld bioprinters are manually controlled by the surgeon. One such bioprinter has already been used by a surgeon for in vivo wound dressing.
Affordable and DIY inkjet bioprinters
The inkjet bioprinting systems typically use one of two drop-generation technologies. The first is piezoelectric technology, in which a piezoelectric actuator is used as a droplet generator. The second is high-speed microsolenoid valve technology for droplet generation, in which droplets are formed through the nozzle through the combination of the syringe pump and the micro-solenoid valve attached to the print head.
In fact, there are not many low-cost inkjet bioprinters and FUJIFILM’s Dimatix’s DMP-2850 is one of the few in this category. It has a piezo-driven jetting device with usable ink capacity of 1.5 ml. For basic research in bioprinting, it is important to be able to work with small volumes of a few milliliters. The cartridge consists of a row with 12 nozzles and each can produce a drop with a volume of 2.4 pL and a size as small as 30 µm. The other bioprinters in this category are MicroFab’s tabletop jetlab 4 and the larger area jetlab 4xl.
Similarly, in terms of DIY inkjet bioprinters, only two open source reports have been identified with price tags of $150 and $325. The latter describes the conversion of an HP DeskJet 500 printer into a bioprinter. The relatively straightforward approach can be translated to other inkjet printers but to avoid frequent clogging of the nozzle, older generations of inkjet printers with a resolution of 300 DPI up to 600 DPI have been recommended. Small volumes of bioink of about 100 µl can be used and the droplet volume was reported to be about 130 picolitres. However, this report demonstrated printing of only one-layer droplets containing cells and its applicability for the construction of 3D objects remains to be shown.
Affordable and DIY stereolithographic bioprinters
A stereolithographic (SLA), or vat polymerization-based, bioprinter utilizes bioinks that are light-sensitive and are cross-linked (solidified) when exposed to a particular wavelength of light. SLA was the first additive manufacturing-based technique. In most cases, the bioinks are placed inside a vat and are sequentially exposed, point-by-point (true SLA) or layer-by-layer (e.g. digital light processing, DLP), from a light source to form 3D objects. Recently, volumetric additive manufacturing (VAM), a layerless technique, was demonstrated. Typically, SLA utilizes an ultraviolet (UV) laser while DLP utilizes a projector as the light source. Masked SLA or LCD SLA printers are the most affordable in this class since they employ an LED source with an LCD screen, acting as a mask, however, it is more limited regarding resolution and printing quality. Finally, VAM directly creates structures in 3D without curing the material voxel by voxel or layer by layer but rather, in its more recent implementation, on a rotating stage, using a DLP-based projector, the bioink is sequentially exposed to projected images which enables the tomographic reconstruction of an object.
Similar to the inkjet bioprinters, there are only few SLA bioprinters on the market. Still, CELLINK’s Lumen X+ and Allegro 3D’s STEMAKER are relatively accessible but our research shows that they are above the $10,000 mark. Both are DLP-based bioprinters with orders-of-magnitude-faster printing speed and better resolution than extrusion and inkjet bioprinters. They use a 405 nm light source to minimize UV damage to cells. Notably, the STEMAKER is capable of printing in 6, 12, and 24-well plates in a temperature-controlled environment.
Another bioprinter which is relatively accessible but still above the $10,000 mark is Tissuelab’s TissueRay. The bioprinter is reportedly priced at ~$14,000 and the first Masked SLA, or LCD SLA bioprinter on the market. It comes equipped with a 4K LCD screen limiting the print resolution at 35 μm and can print in Petri dishes. An intriguing aspect of the bioprinter is its customizable light source. While the 405 nm light source is common in SLA bioprinting, its energy is still high enough to cause damage to cells during bioprinting. Thus, utilizing a light source with a longer wavelength opens up possibilities for low cell damage SLA bioprinting.
There are a couple of projects describing open source DIY stereolithographic bioprinters. According to Garciamendez-Mijares and colleagues’ estimates, these projects cost about $1500 and $4000, respectively. The more affordable of the two consists of a common commercial beam projector, Acer, a water filter to block the harmful infrared radiation, and a petri dish in which the polymerization occurs. The reported resolution of the setup in the plane is as low as 50 μm with curing time per layer of 2 min. However, it remains unclear how each layer is defined given the transparency of the bioresin which presumes light penetration through the whole volume of the liquid. In a related challenge, it is not obvious how this system can create a structure with embedded channels which is important when creating realistic biomimetic vascularized tissue models. Nevertheless, such simple and accessible systems have an important role to play in enabling the development of variety of bioresins suitable for stereolithography which otherwise, require the purchase of a not-so-cheap SLA bioprinter. Not only that but given the lack of SLA bioprinters utilizing a white light source, such a system enables the development of bioresins which are expected to be less damaging to cells.
It is exciting when the first report of an innovative technology is shared open source. VAM is currently the fastest additive manufacturing method where an object which otherwise may take hours to print is created within minutes. The publication of Kelly and colleagues in Science may be paid but the accompanying supplementary material, which is free of charge, contains a lot of the details needed to reproduce their approach. Furthermore, the authors have shared their code on GitHub which makes it even more accessible for anyone enthusiastic about VAM. The setup includes a DLP projection system and a cylindrical glass vial, containing the resin, on a motorized precision rotation stage, Thorlabs PRM1Z8. In spite of the fact that no cells were included in the hydrogels and thus, strictly speaking, no bioprinting was demonstrated, in fact, the report utilized GelMA, a common polymer for SLA bioprinting, and cell-compatible photoinitiators and light. Notably, the fast solidification process and the compatibility of the method with high viscosity formulations would help avoid a common problem in vat polymerization bioprinting where the cells in a low viscosity bioresin sediment and adhere to the bottom of the vat resulting in a non-uniform cell distribution within a printed construct.
Challenges accompanying the use of low cost and DiY bioprinters
One of the main challenges for the assembly of an open-source bioprinter or working with a low cost bioprinter is the need for highly technical and specialized knowledge. The required knowledge is in three general areas: engineering, chemistry and biology. Specifically, assembling a DIY bioprinter requires background in mechanical engineering, electronics, and programming. Insufficient expertise in any or each of these fields will make printing a frustrating experience. Another potential source of frustration can be the improper selection or formulation of a bioink which requires considerable knowledge in physics, chemistry, and biology. It may include a multiple-days long synthesis whose specific procedure will determine the mechanical, rheological, chemical, and biological properties of the bioink. Moreover, proper cell culturing and cell handling techniques are critical for the successful creation of an in vitro tissue model. Finally, choosing appropriate printing parameters usually requires extensive experience with bioprinting. Familiarity with the impact of various parameters, including head and bed temperature, curing dose, flow rate, printing velocity, pressure, on the final result is often decisive for a successful biofabrication.
In spite of the wealth of open source information, the reality is that sharing these types of specialized knowledge is rare which slows down the process of technological democratization. For instance, it is still difficult to find a course on bioprinting at universities. A solution is to organize bioprinting classes and workshops in universities and makerspace labs, as well as to include such classes and courses as part of the academic curricula offered to students. In particular, hackerspace and makerspace labs are especially suitable for collaborative teachings which give the public more opportunities to have access to specialized knowledge and can thus develop a strong DIY community.
The exponential progress in bioprinting has introduced new methods for tissue engineering but it is still a relatively new and developing field. Thus, the DIY bioprinting community can play a significant role in the technological democratization by enabling groups around the world to create tissue models and artificial organs for a fraction of the typical cost. Since bioprinting, most likely, will not be the silver bullet of tissue engineering but it will be a part of a mix of biofabrication approaches, including electrospinning and single cell dispensing, it seems advantageous to bring together individuals with diverse backgrounds, expertise, and aspirations to facilitate the success of this emerging field.